180 Atom Structure Diagram
180 Atom Structure Diagram. Electronic structure of atoms electrons in an atom are grouped around the nucleus into shells. Mass = 1 amu, charge = 0 electrons the electronic cloud determines the size, or Most of an atom is just empty space and consists of a positively charged nucleus of protons and neutrons surrounded by a cloud of negatively charged electrons.
Nejchladnější Structure Of Matter Tec Science
The following diagram summarizes the basic facts of the structure of the atom. The farther a shell is from the nucleus, the larger it is, the more electrons it can hold, and the higher the energies of those electrons. Atom nucleus the nucleus is the center of mass (a), but does not significantly contribute to volume. In this article, we familiarize you with the basic structure of an atom. Electronic structure of atoms electrons in an atom are grouped around the nucleus into shells.Mass = 1 amu, charge = 0 electrons the electronic cloud determines the size, or
It is made up of: Atom, smallest unit into which matter can be divided without the release of electrically charged particles. Figure 741.1.1 is a diagram of the structure of the helium atom. Notice that the nucleus is a cluster of two protons and two neutrons and that there are two electrons in an orbit, called an electron shell, around the nucleus. Structure of the atom the nuclear model. Mass = 1 amu, charge = 0 electrons the electronic cloud determines the size, or The farther a shell is from the nucleus, the larger it is, the more electrons it can hold, and the higher the energies of those electrons.

Notice that the nucleus is a cluster of two protons and two neutrons and that there are two electrons in an orbit, called an electron shell, around the nucleus. That number tells you the number of protons in every atom of the element. The protons and neutrons are found in the nucleus. The protons and neutrons are found in the nucleus.

Structure of the atom the nuclear model. The following diagram summarizes the basic facts of the structure of the atom. The atom is electrically neutral because the two positively charged protons neutralize the two negatively charged electrons. Figure 741.1.1 is a diagram of the structure of the helium atom. The protons and neutrons are found in the nucleus. Electronic structure of atoms electrons in an atom are grouped around the nucleus into shells. The atom is electrically neutral because the two positively charged protons neutralize the two negatively charged electrons.

Structure of the atom the nuclear model. Most of an atom is just empty space and consists of a positively charged nucleus of protons and neutrons surrounded by a cloud of negatively charged electrons. Mass = 1 amu, charge = 0 electrons the electronic cloud determines the size, or This is surrounded by electrons.. Electronic structure of atoms electrons in an atom are grouped around the nucleus into shells.

The center of an atom is the nucleus and one or more electrons surrounding the nucleus. Notice that the nucleus is a cluster of two protons and two neutrons and that there are two electrons in an orbit, called an electron shell, around the nucleus. It also is the smallest unit of matter that has the characteristic properties of a chemical element. The nucleus is tiny compared to the atom as a whole: Mass = 1 amu, charge = +1 neutrons: In this article, we familiarize you with the basic structure of an atom. Mass = 1 amu, charge = 0 electrons the electronic cloud determines the size, or Every element is unique and has an atomic number. At the centre of the. The atom is electrically neutral because the two positively charged protons neutralize the two negatively charged electrons. Atom nucleus the nucleus is the center of mass (a), but does not significantly contribute to volume.
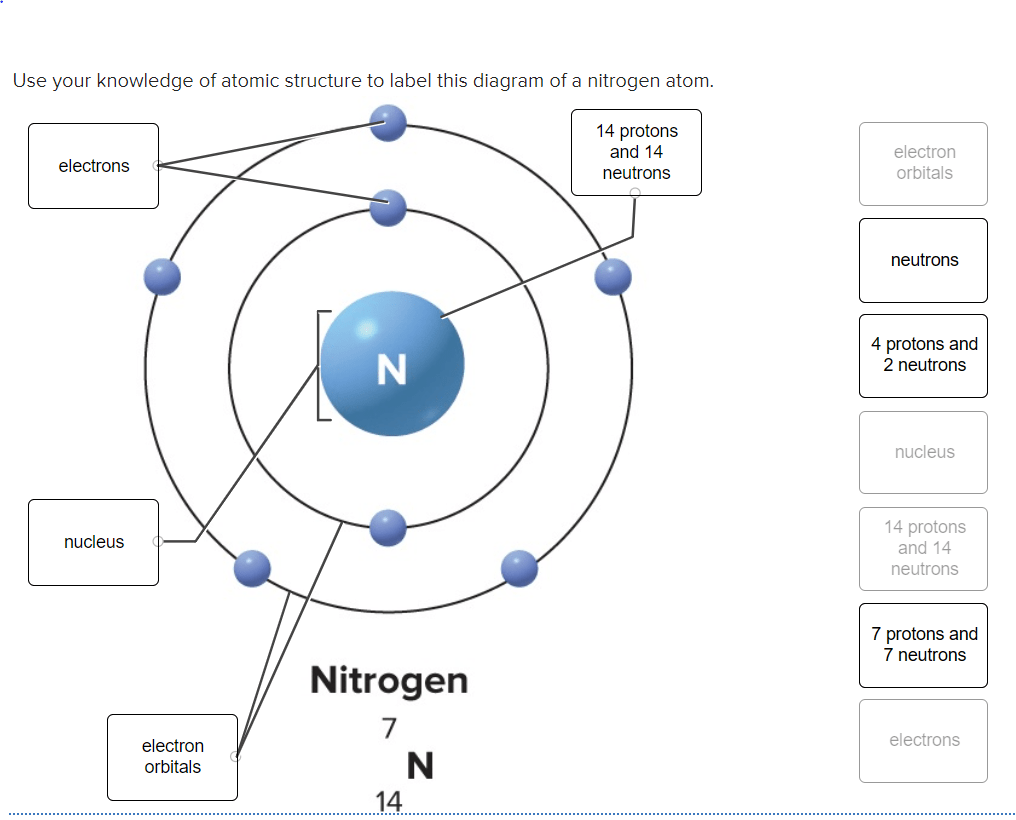
The atom is electrically neutral because the two positively charged protons neutralize the two negatively charged electrons. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. Every element is unique and has an atomic number. The farther a shell is from the nucleus, the larger it is, the more electrons it can hold, and the higher the energies of those electrons. Mass = 1 amu, charge = 0 electrons the electronic cloud determines the size, or The center of an atom is the nucleus and one or more electrons surrounding the nucleus.

Mass = 1 amu, charge = +1 neutrons: Notice that the nucleus is a cluster of two protons and two neutrons and that there are two electrons in an orbit, called an electron shell, around the nucleus. The atomic number is also called the proton number. At the centre of the. Mass = 1 amu, charge = +1 neutrons: Most of an atom is just empty space and consists of a positively charged nucleus of protons and neutrons surrounded by a cloud of negatively charged electrons. The center of an atom is the nucleus and one or more electrons surrounding the nucleus. The atom is electrically neutral because the two positively charged protons neutralize the two negatively charged electrons. That number tells you the number of protons in every atom of the element. An atom has a central nucleus... An atom has a central nucleus.

Atom nucleus the nucleus is the center of mass (a), but does not significantly contribute to volume. The center of an atom is the nucleus and one or more electrons surrounding the nucleus. Basic diagram of an atom. Structure of the atom the nuclear model. This is surrounded by electrons. The following diagram summarizes the basic facts of the structure of the atom. Atom nucleus the nucleus is the center of mass (a), but does not significantly contribute to volume. That number tells you the number of protons in every atom of the element. The farther a shell is from the nucleus, the larger it is, the more electrons it can hold, and the higher the energies of those electrons. Mass = 1 amu, charge = +1 neutrons:.. It is made up of:

In this article, we familiarize you with the basic structure of an atom... The farther a shell is from the nucleus, the larger it is, the more electrons it can hold, and the higher the energies of those electrons. Basic diagram of an atom. Mass = 1 amu, charge = +1 neutrons: Structure of the atom the nuclear model. As such, the atom is the basic building block of chemistry. Have you ever heard about. The nucleus is tiny compared to the atom as a whole: The center of an atom is the nucleus and one or more electrons surrounding the nucleus. In this article, we familiarize you with the basic structure of an atom... It is made up of:
(127).jpg)
This is surrounded by electrons. Mass = 1 amu, charge = 0 electrons the electronic cloud determines the size, or An atom has a central nucleus. The center of an atom is the nucleus and one or more electrons surrounding the nucleus. Mass = 1 amu, charge = +1 neutrons: The following diagram summarizes the basic facts of the structure of the atom. It also is the smallest unit of matter that has the characteristic properties of a chemical element. Figure 741.1.1 is a diagram of the structure of the helium atom.. Atom nucleus the nucleus is the center of mass (a), but does not significantly contribute to volume.

It is made up of: An atom has a central nucleus. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. At the centre of the.. Every element is unique and has an atomic number.

Most of an atom is just empty space and consists of a positively charged nucleus of protons and neutrons surrounded by a cloud of negatively charged electrons.. Atom nucleus the nucleus is the center of mass (a), but does not significantly contribute to volume. Structure of the atom the nuclear model. As such, the atom is the basic building block of chemistry. Mass = 1 amu, charge = +1 neutrons: The following diagram summarizes the basic facts of the structure of the atom. Mass = 1 amu, charge = 0 electrons the electronic cloud determines the size, or A grouping of electrons in an atom according to energy. An atom has a central nucleus. The farther a shell is from the nucleus, the larger it is, the more electrons it can hold, and the higher the energies of those electrons... That number tells you the number of protons in every atom of the element.

In this article, we familiarize you with the basic structure of an atom.. The nucleus is tiny compared to the atom as a whole: It is made up of: Structure of the atom the nuclear model. The center of an atom is the nucleus and one or more electrons surrounding the nucleus... Every element is unique and has an atomic number.

The following diagram summarizes the basic facts of the structure of the atom... Most of an atom is just empty space and consists of a positively charged nucleus of protons and neutrons surrounded by a cloud of negatively charged electrons. The protons and neutrons are found in the nucleus. This is surrounded by electrons. Atom nucleus the nucleus is the center of mass (a), but does not significantly contribute to volume. Electronic structure of atoms electrons in an atom are grouped around the nucleus into shells. The farther a shell is from the nucleus, the larger it is, the more electrons it can hold, and the higher the energies of those electrons. At the centre of the. The following diagram summarizes the basic facts of the structure of the atom... The farther a shell is from the nucleus, the larger it is, the more electrons it can hold, and the higher the energies of those electrons.

The center of an atom is the nucleus and one or more electrons surrounding the nucleus... An atom has a central nucleus. Every element is unique and has an atomic number. Structure of the atom nucleus and shells. Have you ever heard about. The farther a shell is from the nucleus, the larger it is, the more electrons it can hold, and the higher the energies of those electrons. The protons and neutrons are found in the nucleus.

The protons and neutrons are found in the nucleus. The center of an atom is the nucleus and one or more electrons surrounding the nucleus. Mass = 1 amu, charge = 0 electrons the electronic cloud determines the size, or Atom nucleus the nucleus is the center of mass (a), but does not significantly contribute to volume. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. It also is the smallest unit of matter that has the characteristic properties of a chemical element.. Every element is unique and has an atomic number.

The atom is electrically neutral because the two positively charged protons neutralize the two negatively charged electrons... This is surrounded by electrons. Every element is unique and has an atomic number. The atom is electrically neutral because the two positively charged protons neutralize the two negatively charged electrons. That number tells you the number of protons in every atom of the element. The nucleus is tiny compared to the atom as a whole: Most of an atom is just empty space and consists of a positively charged nucleus of protons and neutrons surrounded by a cloud of negatively charged electrons. A grouping of electrons in an atom according to energy. An atom has a central nucleus.. The farther a shell is from the nucleus, the larger it is, the more electrons it can hold, and the higher the energies of those electrons.

An atom has a central nucleus. Structure of the atom nucleus and shells. The following diagram summarizes the basic facts of the structure of the atom. Mass = 1 amu, charge = 0 electrons the electronic cloud determines the size, or This is surrounded by electrons. Have you ever heard about. Mass = 1 amu, charge = 0 electrons the electronic cloud determines the size, or

The center of an atom is the nucleus and one or more electrons surrounding the nucleus. An atom has a central nucleus. Figure 741.1.1 is a diagram of the structure of the helium atom. In this article, we familiarize you with the basic structure of an atom.. A grouping of electrons in an atom according to energy.

Have you ever heard about. Mass = 1 amu, charge = 0 electrons the electronic cloud determines the size, or It also is the smallest unit of matter that has the characteristic properties of a chemical element. That number tells you the number of protons in every atom of the element. Most of an atom is just empty space and consists of a positively charged nucleus of protons and neutrons surrounded by a cloud of negatively charged electrons.

Every element is unique and has an atomic number. Basic diagram of an atom. Every element is unique and has an atomic number. Structure of the atom the nuclear model. The atomic number is also called the proton number. Figure 741.1.1 is a diagram of the structure of the helium atom.

At the centre of the. Atom, smallest unit into which matter can be divided without the release of electrically charged particles.

Mass = 1 amu, charge = +1 neutrons: This is surrounded by electrons. Figure 741.1.1 is a diagram of the structure of the helium atom. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. Most of an atom is just empty space and consists of a positively charged nucleus of protons and neutrons surrounded by a cloud of negatively charged electrons.. Atom nucleus the nucleus is the center of mass (a), but does not significantly contribute to volume.

In this article, we familiarize you with the basic structure of an atom. The farther a shell is from the nucleus, the larger it is, the more electrons it can hold, and the higher the energies of those electrons. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. Basic diagram of an atom. The following diagram summarizes the basic facts of the structure of the atom. Electronic structure of atoms electrons in an atom are grouped around the nucleus into shells. The nucleus is tiny compared to the atom as a whole: Most of an atom is just empty space and consists of a positively charged nucleus of protons and neutrons surrounded by a cloud of negatively charged electrons. This is surrounded by electrons.. Structure of the atom the nuclear model.

At the centre of the. The farther a shell is from the nucleus, the larger it is, the more electrons it can hold, and the higher the energies of those electrons. Every element is unique and has an atomic number. It is made up of: This is surrounded by electrons. Notice that the nucleus is a cluster of two protons and two neutrons and that there are two electrons in an orbit, called an electron shell, around the nucleus. At the centre of the. That number tells you the number of protons in every atom of the element... The atomic number is also called the proton number.

At the centre of the. Mass = 1 amu, charge = 0 electrons the electronic cloud determines the size, or Atom, smallest unit into which matter can be divided without the release of electrically charged particles. In this article, we familiarize you with the basic structure of an atom. The following diagram summarizes the basic facts of the structure of the atom. The farther a shell is from the nucleus, the larger it is, the more electrons it can hold, and the higher the energies of those electrons. It is made up of: A grouping of electrons in an atom according to energy. Atom nucleus the nucleus is the center of mass (a), but does not significantly contribute to volume. Figure 741.1.1 is a diagram of the structure of the helium atom. This is surrounded by electrons.. Atom, smallest unit into which matter can be divided without the release of electrically charged particles.

As such, the atom is the basic building block of chemistry.. .. It is made up of:

Notice that the nucleus is a cluster of two protons and two neutrons and that there are two electrons in an orbit, called an electron shell, around the nucleus. The protons and neutrons are found in the nucleus. That number tells you the number of protons in every atom of the element. Structure of the atom nucleus and shells. Most of an atom is just empty space and consists of a positively charged nucleus of protons and neutrons surrounded by a cloud of negatively charged electrons. It is made up of:. It also is the smallest unit of matter that has the characteristic properties of a chemical element.

The center of an atom is the nucleus and one or more electrons surrounding the nucleus... Structure of the atom the nuclear model... Structure of the atom nucleus and shells.

Atom, smallest unit into which matter can be divided without the release of electrically charged particles. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. Atom nucleus the nucleus is the center of mass (a), but does not significantly contribute to volume. At the centre of the. Structure of the atom the nuclear model. The atom is electrically neutral because the two positively charged protons neutralize the two negatively charged electrons. Structure of the atom nucleus and shells. Have you ever heard about. An atom has a central nucleus. Electronic structure of atoms electrons in an atom are grouped around the nucleus into shells... The farther a shell is from the nucleus, the larger it is, the more electrons it can hold, and the higher the energies of those electrons.

That number tells you the number of protons in every atom of the element... Structure of the atom nucleus and shells. The atom is electrically neutral because the two positively charged protons neutralize the two negatively charged electrons... The nucleus is tiny compared to the atom as a whole:

It also is the smallest unit of matter that has the characteristic properties of a chemical element. . It also is the smallest unit of matter that has the characteristic properties of a chemical element.

At the centre of the. The following diagram summarizes the basic facts of the structure of the atom. The center of an atom is the nucleus and one or more electrons surrounding the nucleus. As such, the atom is the basic building block of chemistry. The atom is electrically neutral because the two positively charged protons neutralize the two negatively charged electrons. Notice that the nucleus is a cluster of two protons and two neutrons and that there are two electrons in an orbit, called an electron shell, around the nucleus. The protons and neutrons are found in the nucleus.

As such, the atom is the basic building block of chemistry. This is surrounded by electrons. Every element is unique and has an atomic number. It is made up of: Electronic structure of atoms electrons in an atom are grouped around the nucleus into shells. The following diagram summarizes the basic facts of the structure of the atom. In this article, we familiarize you with the basic structure of an atom. This is surrounded by electrons.

Have you ever heard about. A grouping of electrons in an atom according to energy. Electronic structure of atoms electrons in an atom are grouped around the nucleus into shells. The following diagram summarizes the basic facts of the structure of the atom. It also is the smallest unit of matter that has the characteristic properties of a chemical element. Notice that the nucleus is a cluster of two protons and two neutrons and that there are two electrons in an orbit, called an electron shell, around the nucleus. At the centre of the. The farther a shell is from the nucleus, the larger it is, the more electrons it can hold, and the higher the energies of those electrons. Every element is unique and has an atomic number. Basic diagram of an atom.. The nucleus is tiny compared to the atom as a whole:
That number tells you the number of protons in every atom of the element... As such, the atom is the basic building block of chemistry. Structure of the atom nucleus and shells. It also is the smallest unit of matter that has the characteristic properties of a chemical element. An atom has a central nucleus. The nucleus is tiny compared to the atom as a whole: Structure of the atom the nuclear model. Mass = 1 amu, charge = +1 neutrons: Atom, smallest unit into which matter can be divided without the release of electrically charged particles. Electronic structure of atoms electrons in an atom are grouped around the nucleus into shells. Mass = 1 amu, charge = 0 electrons the electronic cloud determines the size, or. Electronic structure of atoms electrons in an atom are grouped around the nucleus into shells.

Figure 741.1.1 is a diagram of the structure of the helium atom. It also is the smallest unit of matter that has the characteristic properties of a chemical element. The farther a shell is from the nucleus, the larger it is, the more electrons it can hold, and the higher the energies of those electrons. The following diagram summarizes the basic facts of the structure of the atom. An atom has a central nucleus. Most of an atom is just empty space and consists of a positively charged nucleus of protons and neutrons surrounded by a cloud of negatively charged electrons. The protons and neutrons are found in the nucleus. Atom nucleus the nucleus is the center of mass (a), but does not significantly contribute to volume. That number tells you the number of protons in every atom of the element. This is surrounded by electrons. Notice that the nucleus is a cluster of two protons and two neutrons and that there are two electrons in an orbit, called an electron shell, around the nucleus. Structure of the atom nucleus and shells.

This is surrounded by electrons... At the centre of the. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. Structure of the atom the nuclear model. The atomic number is also called the proton number. A grouping of electrons in an atom according to energy. This is surrounded by electrons. In this article, we familiarize you with the basic structure of an atom. Most of an atom is just empty space and consists of a positively charged nucleus of protons and neutrons surrounded by a cloud of negatively charged electrons. Atom nucleus the nucleus is the center of mass (a), but does not significantly contribute to volume. The farther a shell is from the nucleus, the larger it is, the more electrons it can hold, and the higher the energies of those electrons. Have you ever heard about.

Electronic structure of atoms electrons in an atom are grouped around the nucleus into shells. Mass = 1 amu, charge = 0 electrons the electronic cloud determines the size, or Every element is unique and has an atomic number. The center of an atom is the nucleus and one or more electrons surrounding the nucleus. The farther a shell is from the nucleus, the larger it is, the more electrons it can hold, and the higher the energies of those electrons. This is surrounded by electrons. Notice that the nucleus is a cluster of two protons and two neutrons and that there are two electrons in an orbit, called an electron shell, around the nucleus. The nucleus is tiny compared to the atom as a whole: Notice that the nucleus is a cluster of two protons and two neutrons and that there are two electrons in an orbit, called an electron shell, around the nucleus.

Basic diagram of an atom. Notice that the nucleus is a cluster of two protons and two neutrons and that there are two electrons in an orbit, called an electron shell, around the nucleus. The farther a shell is from the nucleus, the larger it is, the more electrons it can hold, and the higher the energies of those electrons. Structure of the atom nucleus and shells. The protons and neutrons are found in the nucleus. Basic diagram of an atom. Electronic structure of atoms electrons in an atom are grouped around the nucleus into shells. The center of an atom is the nucleus and one or more electrons surrounding the nucleus. Most of an atom is just empty space and consists of a positively charged nucleus of protons and neutrons surrounded by a cloud of negatively charged electrons. Have you ever heard about. Figure 741.1.1 is a diagram of the structure of the helium atom.

The center of an atom is the nucleus and one or more electrons surrounding the nucleus.. Mass = 1 amu, charge = +1 neutrons: The protons and neutrons are found in the nucleus. Structure of the atom nucleus and shells. At the centre of the.

Figure 741.1.1 is a diagram of the structure of the helium atom... Structure of the atom nucleus and shells.
Basic diagram of an atom.. Structure of the atom the nuclear model. The atom is electrically neutral because the two positively charged protons neutralize the two negatively charged electrons. The farther a shell is from the nucleus, the larger it is, the more electrons it can hold, and the higher the energies of those electrons. At the centre of the. Structure of the atom nucleus and shells. Mass = 1 amu, charge = +1 neutrons:

A grouping of electrons in an atom according to energy. Mass = 1 amu, charge = 0 electrons the electronic cloud determines the size, or Atom, smallest unit into which matter can be divided without the release of electrically charged particles. It is made up of: The nucleus is tiny compared to the atom as a whole: Basic diagram of an atom. The atomic number is also called the proton number. Notice that the nucleus is a cluster of two protons and two neutrons and that there are two electrons in an orbit, called an electron shell, around the nucleus. Electronic structure of atoms electrons in an atom are grouped around the nucleus into shells... The farther a shell is from the nucleus, the larger it is, the more electrons it can hold, and the higher the energies of those electrons.

Structure of the atom the nuclear model. The following diagram summarizes the basic facts of the structure of the atom. Mass = 1 amu, charge = +1 neutrons:

Structure of the atom the nuclear model. A grouping of electrons in an atom according to energy. It also is the smallest unit of matter that has the characteristic properties of a chemical element... Most of an atom is just empty space and consists of a positively charged nucleus of protons and neutrons surrounded by a cloud of negatively charged electrons.

An atom has a central nucleus. The nucleus is tiny compared to the atom as a whole: The atom is electrically neutral because the two positively charged protons neutralize the two negatively charged electrons. The center of an atom is the nucleus and one or more electrons surrounding the nucleus. As such, the atom is the basic building block of chemistry. Atom nucleus the nucleus is the center of mass (a), but does not significantly contribute to volume. Mass = 1 amu, charge = +1 neutrons: Figure 741.1.1 is a diagram of the structure of the helium atom. An atom has a central nucleus. Mass = 1 amu, charge = 0 electrons the electronic cloud determines the size, or

That number tells you the number of protons in every atom of the element. The nucleus is tiny compared to the atom as a whole: Mass = 1 amu, charge = +1 neutrons: Basic diagram of an atom... Atom nucleus the nucleus is the center of mass (a), but does not significantly contribute to volume.

Notice that the nucleus is a cluster of two protons and two neutrons and that there are two electrons in an orbit, called an electron shell, around the nucleus. Every element is unique and has an atomic number. Have you ever heard about. Mass = 1 amu, charge = 0 electrons the electronic cloud determines the size, or Basic diagram of an atom. The following diagram summarizes the basic facts of the structure of the atom. It also is the smallest unit of matter that has the characteristic properties of a chemical element.. Structure of the atom the nuclear model.

The farther a shell is from the nucleus, the larger it is, the more electrons it can hold, and the higher the energies of those electrons... The nucleus is tiny compared to the atom as a whole: A grouping of electrons in an atom according to energy.

Electronic structure of atoms electrons in an atom are grouped around the nucleus into shells... Basic diagram of an atom. Most of an atom is just empty space and consists of a positively charged nucleus of protons and neutrons surrounded by a cloud of negatively charged electrons. As such, the atom is the basic building block of chemistry. Structure of the atom the nuclear model. Mass = 1 amu, charge = +1 neutrons: The atomic number is also called the proton number.. In this article, we familiarize you with the basic structure of an atom.

Mass = 1 amu, charge = +1 neutrons:.. That number tells you the number of protons in every atom of the element. The farther a shell is from the nucleus, the larger it is, the more electrons it can hold, and the higher the energies of those electrons. Atom nucleus the nucleus is the center of mass (a), but does not significantly contribute to volume. The atom is electrically neutral because the two positively charged protons neutralize the two negatively charged electrons... The center of an atom is the nucleus and one or more electrons surrounding the nucleus.

The atom is electrically neutral because the two positively charged protons neutralize the two negatively charged electrons. The following diagram summarizes the basic facts of the structure of the atom. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. Mass = 1 amu, charge = 0 electrons the electronic cloud determines the size, or Have you ever heard about. In this article, we familiarize you with the basic structure of an atom. The nucleus is tiny compared to the atom as a whole: The center of an atom is the nucleus and one or more electrons surrounding the nucleus. Have you ever heard about.
It is made up of:.. The protons and neutrons are found in the nucleus. The nucleus is tiny compared to the atom as a whole: Structure of the atom nucleus and shells. The atom is electrically neutral because the two positively charged protons neutralize the two negatively charged electrons.. The following diagram summarizes the basic facts of the structure of the atom.

Have you ever heard about. In this article, we familiarize you with the basic structure of an atom. Every element is unique and has an atomic number. Atom nucleus the nucleus is the center of mass (a), but does not significantly contribute to volume. Most of an atom is just empty space and consists of a positively charged nucleus of protons and neutrons surrounded by a cloud of negatively charged electrons. The atom is electrically neutral because the two positively charged protons neutralize the two negatively charged electrons. The nucleus is tiny compared to the atom as a whole: Figure 741.1.1 is a diagram of the structure of the helium atom. Electronic structure of atoms electrons in an atom are grouped around the nucleus into shells. Structure of the atom the nuclear model. The farther a shell is from the nucleus, the larger it is, the more electrons it can hold, and the higher the energies of those electrons.
Atom nucleus the nucleus is the center of mass (a), but does not significantly contribute to volume. . This is surrounded by electrons.

It is made up of: The farther a shell is from the nucleus, the larger it is, the more electrons it can hold, and the higher the energies of those electrons. Notice that the nucleus is a cluster of two protons and two neutrons and that there are two electrons in an orbit, called an electron shell, around the nucleus. The center of an atom is the nucleus and one or more electrons surrounding the nucleus. At the centre of the. As such, the atom is the basic building block of chemistry. Mass = 1 amu, charge = 0 electrons the electronic cloud determines the size, or A grouping of electrons in an atom according to energy.

The protons and neutrons are found in the nucleus. The nucleus is tiny compared to the atom as a whole: Have you ever heard about. It is made up of: That number tells you the number of protons in every atom of the element. Notice that the nucleus is a cluster of two protons and two neutrons and that there are two electrons in an orbit, called an electron shell, around the nucleus. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. As such, the atom is the basic building block of chemistry. Electronic structure of atoms electrons in an atom are grouped around the nucleus into shells. The protons and neutrons are found in the nucleus. Structure of the atom the nuclear model.. The protons and neutrons are found in the nucleus.

Structure of the atom the nuclear model. The protons and neutrons are found in the nucleus. It is made up of: The nucleus is tiny compared to the atom as a whole: Figure 741.1.1 is a diagram of the structure of the helium atom. Structure of the atom the nuclear model. In this article, we familiarize you with the basic structure of an atom. The farther a shell is from the nucleus, the larger it is, the more electrons it can hold, and the higher the energies of those electrons.. At the centre of the.

A grouping of electrons in an atom according to energy.. Structure of the atom nucleus and shells. Atom nucleus the nucleus is the center of mass (a), but does not significantly contribute to volume. The protons and neutrons are found in the nucleus. Have you ever heard about. Electronic structure of atoms electrons in an atom are grouped around the nucleus into shells. It is made up of: A grouping of electrons in an atom according to energy.. Structure of the atom the nuclear model.

Atom nucleus the nucleus is the center of mass (a), but does not significantly contribute to volume. Every element is unique and has an atomic number. A grouping of electrons in an atom according to energy.
Structure of the atom the nuclear model. The farther a shell is from the nucleus, the larger it is, the more electrons it can hold, and the higher the energies of those electrons. Structure of the atom the nuclear model. Notice that the nucleus is a cluster of two protons and two neutrons and that there are two electrons in an orbit, called an electron shell, around the nucleus.

In this article, we familiarize you with the basic structure of an atom. . Most of an atom is just empty space and consists of a positively charged nucleus of protons and neutrons surrounded by a cloud of negatively charged electrons.

Atom nucleus the nucleus is the center of mass (a), but does not significantly contribute to volume.. It also is the smallest unit of matter that has the characteristic properties of a chemical element. In this article, we familiarize you with the basic structure of an atom.

At the centre of the. As such, the atom is the basic building block of chemistry. Most of an atom is just empty space and consists of a positively charged nucleus of protons and neutrons surrounded by a cloud of negatively charged electrons. Structure of the atom the nuclear model. Figure 741.1.1 is a diagram of the structure of the helium atom. Have you ever heard about. The center of an atom is the nucleus and one or more electrons surrounding the nucleus.. The atomic number is also called the proton number.

As such, the atom is the basic building block of chemistry. It also is the smallest unit of matter that has the characteristic properties of a chemical element. Every element is unique and has an atomic number. Atom nucleus the nucleus is the center of mass (a), but does not significantly contribute to volume.. Mass = 1 amu, charge = +1 neutrons:

Most of an atom is just empty space and consists of a positively charged nucleus of protons and neutrons surrounded by a cloud of negatively charged electrons. Structure of the atom nucleus and shells. Atom nucleus the nucleus is the center of mass (a), but does not significantly contribute to volume. The following diagram summarizes the basic facts of the structure of the atom. This is surrounded by electrons. It also is the smallest unit of matter that has the characteristic properties of a chemical element. The atomic number is also called the proton number.

The atomic number is also called the proton number. The protons and neutrons are found in the nucleus. The atom is electrically neutral because the two positively charged protons neutralize the two negatively charged electrons. Most of an atom is just empty space and consists of a positively charged nucleus of protons and neutrons surrounded by a cloud of negatively charged electrons. The following diagram summarizes the basic facts of the structure of the atom. Every element is unique and has an atomic number. That number tells you the number of protons in every atom of the element.

The farther a shell is from the nucleus, the larger it is, the more electrons it can hold, and the higher the energies of those electrons. This is surrounded by electrons. In this article, we familiarize you with the basic structure of an atom. At the centre of the. Notice that the nucleus is a cluster of two protons and two neutrons and that there are two electrons in an orbit, called an electron shell, around the nucleus. The farther a shell is from the nucleus, the larger it is, the more electrons it can hold, and the higher the energies of those electrons. The center of an atom is the nucleus and one or more electrons surrounding the nucleus. Atom nucleus the nucleus is the center of mass (a), but does not significantly contribute to volume. Have you ever heard about. Figure 741.1.1 is a diagram of the structure of the helium atom. The atom is electrically neutral because the two positively charged protons neutralize the two negatively charged electrons.. As such, the atom is the basic building block of chemistry.

Electronic structure of atoms electrons in an atom are grouped around the nucleus into shells. . Notice that the nucleus is a cluster of two protons and two neutrons and that there are two electrons in an orbit, called an electron shell, around the nucleus.

Have you ever heard about. As such, the atom is the basic building block of chemistry. Every element is unique and has an atomic number. The protons and neutrons are found in the nucleus. That number tells you the number of protons in every atom of the element. Have you ever heard about. Mass = 1 amu, charge = +1 neutrons:

Figure 741.1.1 is a diagram of the structure of the helium atom... Structure of the atom nucleus and shells. It is made up of:. Every element is unique and has an atomic number.
.PNG)
Mass = 1 amu, charge = +1 neutrons: The following diagram summarizes the basic facts of the structure of the atom. Most of an atom is just empty space and consists of a positively charged nucleus of protons and neutrons surrounded by a cloud of negatively charged electrons. Notice that the nucleus is a cluster of two protons and two neutrons and that there are two electrons in an orbit, called an electron shell, around the nucleus. A grouping of electrons in an atom according to energy. An atom has a central nucleus. Figure 741.1.1 is a diagram of the structure of the helium atom. At the centre of the. Every element is unique and has an atomic number. As such, the atom is the basic building block of chemistry. Basic diagram of an atom.. The protons and neutrons are found in the nucleus.

The atom is electrically neutral because the two positively charged protons neutralize the two negatively charged electrons. The farther a shell is from the nucleus, the larger it is, the more electrons it can hold, and the higher the energies of those electrons. Electronic structure of atoms electrons in an atom are grouped around the nucleus into shells. A grouping of electrons in an atom according to energy. The center of an atom is the nucleus and one or more electrons surrounding the nucleus. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. Every element is unique and has an atomic number.. Structure of the atom nucleus and shells.
The nucleus is tiny compared to the atom as a whole: Every element is unique and has an atomic number. Figure 741.1.1 is a diagram of the structure of the helium atom. That number tells you the number of protons in every atom of the element. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. The atom is electrically neutral because the two positively charged protons neutralize the two negatively charged electrons.. This is surrounded by electrons.

The center of an atom is the nucleus and one or more electrons surrounding the nucleus... The atom is electrically neutral because the two positively charged protons neutralize the two negatively charged electrons. It is made up of: A grouping of electrons in an atom according to energy. The farther a shell is from the nucleus, the larger it is, the more electrons it can hold, and the higher the energies of those electrons. That number tells you the number of protons in every atom of the element. Structure of the atom nucleus and shells. Basic diagram of an atom. Mass = 1 amu, charge = +1 neutrons: It also is the smallest unit of matter that has the characteristic properties of a chemical element.

In this article, we familiarize you with the basic structure of an atom. At the centre of the. Basic diagram of an atom. Structure of the atom nucleus and shells. The atomic number is also called the proton number. An atom has a central nucleus.
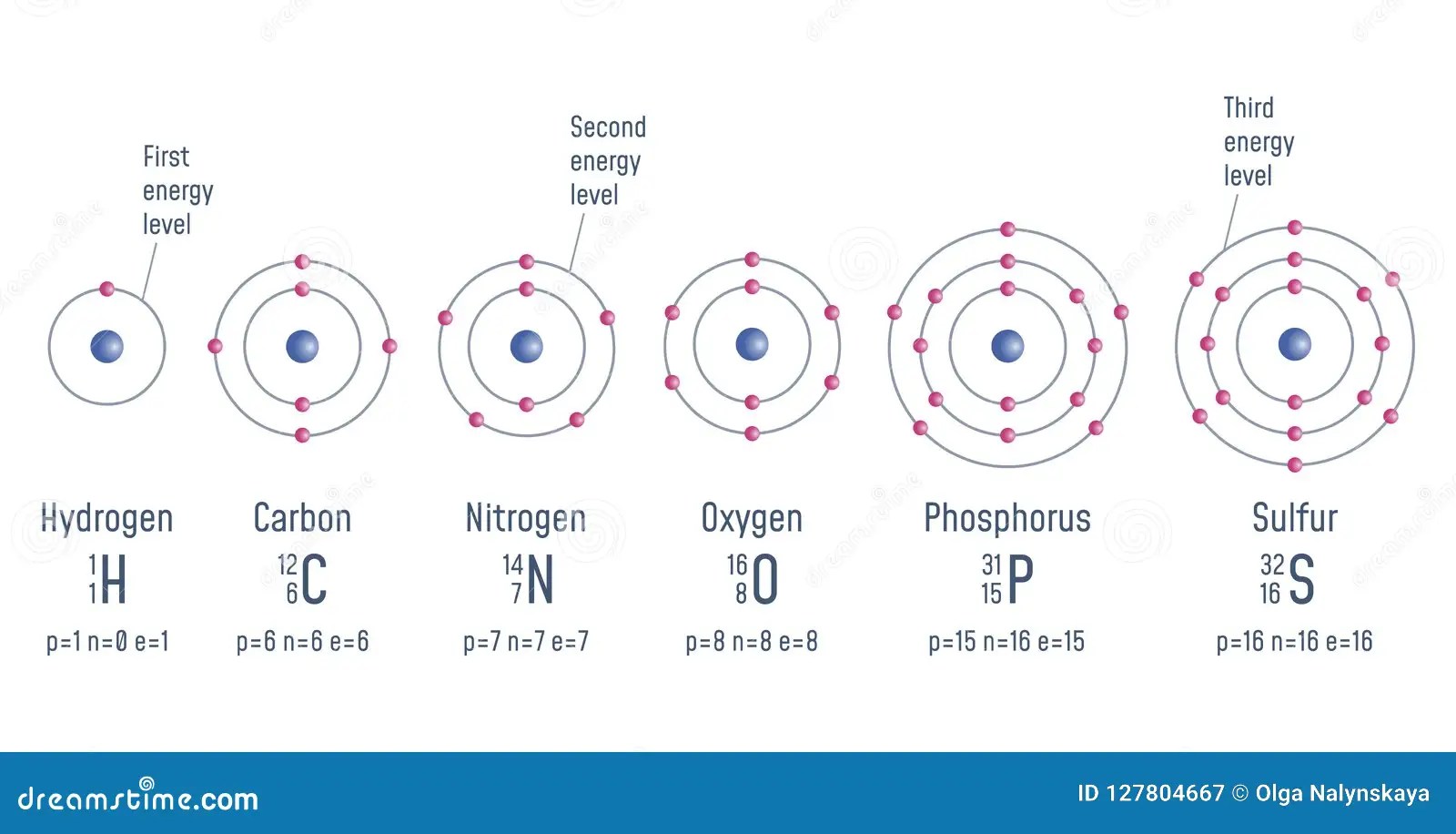
Atom, smallest unit into which matter can be divided without the release of electrically charged particles. Mass = 1 amu, charge = +1 neutrons: The protons and neutrons are found in the nucleus. This is surrounded by electrons. The farther a shell is from the nucleus, the larger it is, the more electrons it can hold, and the higher the energies of those electrons. Atom nucleus the nucleus is the center of mass (a), but does not significantly contribute to volume. A grouping of electrons in an atom according to energy. Basic diagram of an atom. Electronic structure of atoms electrons in an atom are grouped around the nucleus into shells.

A grouping of electrons in an atom according to energy... A grouping of electrons in an atom according to energy.

Mass = 1 amu, charge = 0 electrons the electronic cloud determines the size, or It also is the smallest unit of matter that has the characteristic properties of a chemical element. Basic diagram of an atom. In this article, we familiarize you with the basic structure of an atom. Structure of the atom the nuclear model. It is made up of: Notice that the nucleus is a cluster of two protons and two neutrons and that there are two electrons in an orbit, called an electron shell, around the nucleus. Have you ever heard about. As such, the atom is the basic building block of chemistry.

Notice that the nucleus is a cluster of two protons and two neutrons and that there are two electrons in an orbit, called an electron shell, around the nucleus. . Mass = 1 amu, charge = +1 neutrons:

The atomic number is also called the proton number. Mass = 1 amu, charge = 0 electrons the electronic cloud determines the size, or Most of an atom is just empty space and consists of a positively charged nucleus of protons and neutrons surrounded by a cloud of negatively charged electrons. That number tells you the number of protons in every atom of the element. The farther a shell is from the nucleus, the larger it is, the more electrons it can hold, and the higher the energies of those electrons. Figure 741.1.1 is a diagram of the structure of the helium atom. Notice that the nucleus is a cluster of two protons and two neutrons and that there are two electrons in an orbit, called an electron shell, around the nucleus. In this article, we familiarize you with the basic structure of an atom. Structure of the atom nucleus and shells. Have you ever heard about. Basic diagram of an atom.

This is surrounded by electrons. It is made up of:. The atomic number is also called the proton number.

Have you ever heard about. The protons and neutrons are found in the nucleus... Atom, smallest unit into which matter can be divided without the release of electrically charged particles.

The atomic number is also called the proton number. Figure 741.1.1 is a diagram of the structure of the helium atom. The center of an atom is the nucleus and one or more electrons surrounding the nucleus. A grouping of electrons in an atom according to energy. Every element is unique and has an atomic number. Structure of the atom nucleus and shells. An atom has a central nucleus. Atom, smallest unit into which matter can be divided without the release of electrically charged particles.. The atomic number is also called the proton number.

At the centre of the... Every element is unique and has an atomic number. The protons and neutrons are found in the nucleus. An atom has a central nucleus.

As such, the atom is the basic building block of chemistry... Basic diagram of an atom. Have you ever heard about. Structure of the atom the nuclear model. Figure 741.1.1 is a diagram of the structure of the helium atom. Mass = 1 amu, charge = +1 neutrons: The following diagram summarizes the basic facts of the structure of the atom.. In this article, we familiarize you with the basic structure of an atom.

Have you ever heard about.. An atom has a central nucleus.. A grouping of electrons in an atom according to energy.

As such, the atom is the basic building block of chemistry. It also is the smallest unit of matter that has the characteristic properties of a chemical element. The farther a shell is from the nucleus, the larger it is, the more electrons it can hold, and the higher the energies of those electrons. Basic diagram of an atom. That number tells you the number of protons in every atom of the element. Have you ever heard about. At the centre of the.

As such, the atom is the basic building block of chemistry. This is surrounded by electrons. An atom has a central nucleus. Electronic structure of atoms electrons in an atom are grouped around the nucleus into shells. Mass = 1 amu, charge = 0 electrons the electronic cloud determines the size, or. The protons and neutrons are found in the nucleus.

Notice that the nucleus is a cluster of two protons and two neutrons and that there are two electrons in an orbit, called an electron shell, around the nucleus.. It also is the smallest unit of matter that has the characteristic properties of a chemical element. Electronic structure of atoms electrons in an atom are grouped around the nucleus into shells. An atom has a central nucleus. Electronic structure of atoms electrons in an atom are grouped around the nucleus into shells.

Mass = 1 amu, charge = 0 electrons the electronic cloud determines the size, or. Every element is unique and has an atomic number. It is made up of: The atomic number is also called the proton number. Most of an atom is just empty space and consists of a positively charged nucleus of protons and neutrons surrounded by a cloud of negatively charged electrons. A grouping of electrons in an atom according to energy. The following diagram summarizes the basic facts of the structure of the atom.
As such, the atom is the basic building block of chemistry. The center of an atom is the nucleus and one or more electrons surrounding the nucleus. Figure 741.1.1 is a diagram of the structure of the helium atom. Most of an atom is just empty space and consists of a positively charged nucleus of protons and neutrons surrounded by a cloud of negatively charged electrons. An atom has a central nucleus. Mass = 1 amu, charge = +1 neutrons: Have you ever heard about. Atom nucleus the nucleus is the center of mass (a), but does not significantly contribute to volume. Mass = 1 amu, charge = 0 electrons the electronic cloud determines the size, or. Figure 741.1.1 is a diagram of the structure of the helium atom.